Full Text
Rising atmospheric CO2 and falling ocean pH place an urgency on our efforts to understand the impact of CO2 on Earth’s ecosystems and climate Studies of past perturbations of Earth’s carbon reservoirs and climate—ranging from glacial-interglacial cycles to mass extinction events—may provide valuable insights, but they require the ability to reconstruct changes in ocean-atmosphere CO2 chemistry in Earth’s past. Here, we provide an overview of the boron isotope pH proxy in marine carbonates and how it can be applied to reconstruct past ocean pH and atmospheric CO2.
The Boron Isotope pH Proxy
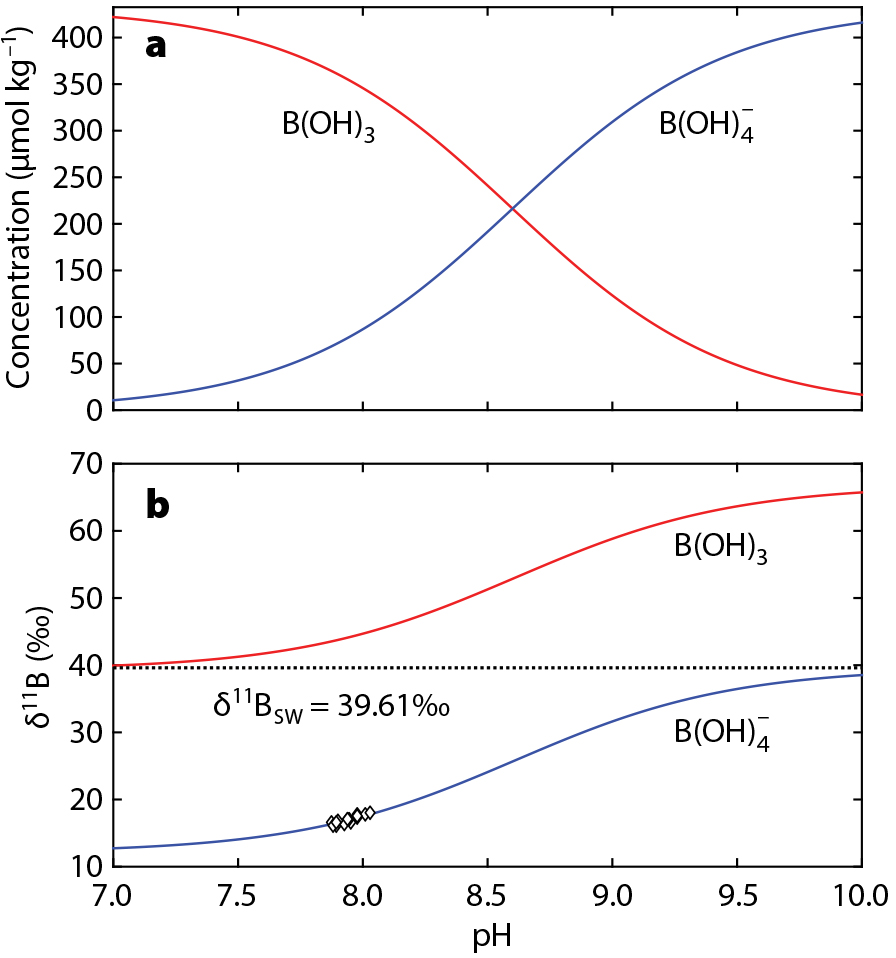
Hemming and Hanson (1992) first suggested using the boron isotopic composition of marine carbonates as a proxy for ocean pH. They proposed a boron isotope pH meter, based on the pH-dependent speciation of the two dominant forms of boron in seawater, boric acid (B(OH)3) and the borate ion (B(OH)4–). At low pH, boric acid dominates and vice versa (Figure 1a). As there is a constant isotopic offset between the two species, the isotopic signature of each shifts as pH changes to conserve mass balance and the overall boron isotope composition of seawater (Figure 1b). Empirical calibrations suggest that marine calcifiers—such as foraminifera, corals, and brachiopods—incorporate the tetrahedral borate ion into their carbonate skeletons (e.g., Rae et al., 2011). As a result, the isotopic composition of fossil CaCO3 may be used to reconstruct that of the borate ion, and in turn pH. While research into the exact mechanism of boron incorporation is ongoing, the original conceptual model described above provides a useful basis for the δ11B pH proxy that is grounded in seawater acid-base chemistry and isotopic equilibria.
|
|
With pH established, another carbonate system parameter is needed to quantitatively reconstruct CO2. Because seawater pH and CO2 are closely coupled, the resulting pCO2 record will be mainly driven by pH. Thus, even a broad estimate of alkalinity can result in a well-constrained pCO2 estimate. The residence time of boron in the ocean is ~10–20 million years, and so changes in seawater δ11B must also be considered when using boron isotopes to reconstruct pH and pCO2 on multimillion-year timescales.
CO2 and pH Change Beyond the Ice Cores
While ice cores provide detailed records of past atmospheric CO2, the records currently only extend back 800,000 years. The boron isotope pH proxy has become one of the key methods paleoceanographers use to extend atmospheric CO2 reconstructions beyond the timescales of ice core records. Recent studies demonstrate coupling between CO2 and long-term climate over the last ~66 million years (e.g., Anagnostou et al., 2020), while on shorter timescales, Martínez-Botí et al. (2015) use δ11B to show that, once differences in ice-albedo feedback are accounted for, climate sensitivity in the Pliocene (~5.3–2.6 million years ago) was similar to modern sensitivity. Boron isotopes have also been applied to examining rapid acidification events, including those associated with carbon release during the Paleocene-Eocene Thermal Maximum (~55 million years ago; Penman et al., 2014) and flash acidification associated with the asteroid impact at the Cretaceous–Paleogene boundary (~66 million years ago; Henehan et al., 2019).
Mechanisms of Glacial-Interglacial CO2 Change
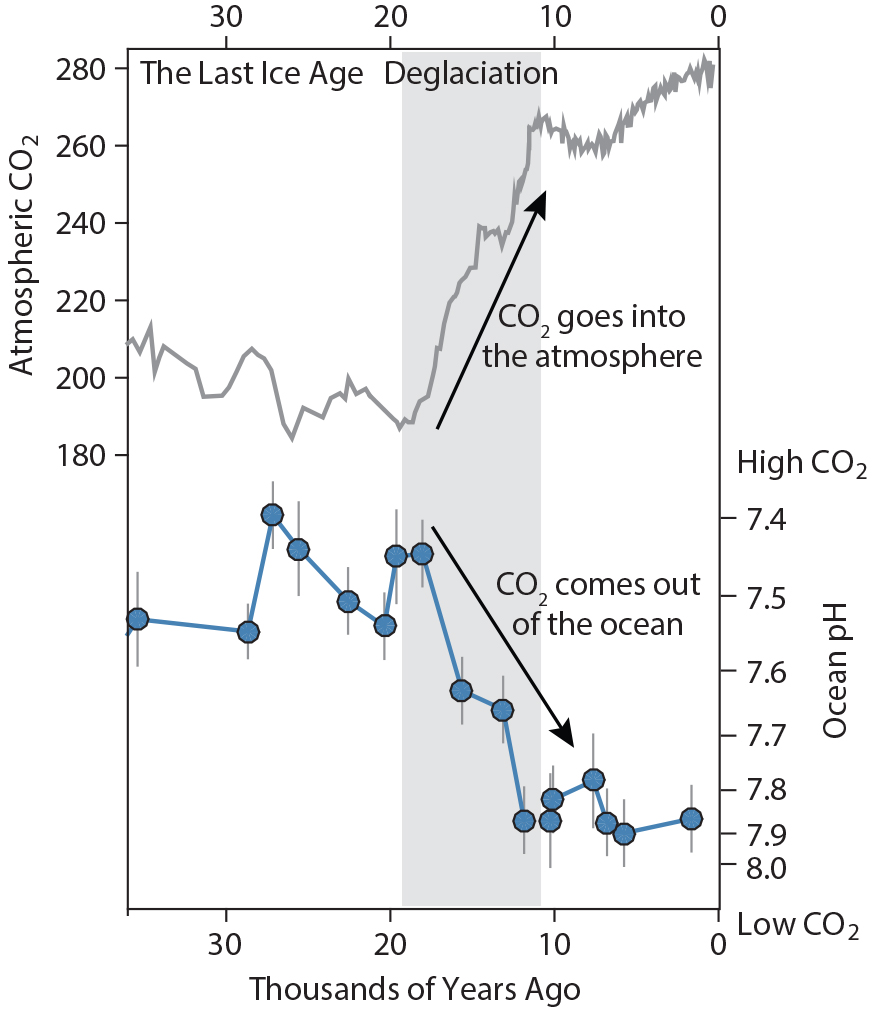
For more recent time periods, boron isotopes can be used to reveal the processes by which CO2 is transferred between the ocean and the atmosphere during glacial-interglacial transitions. Rae et al. (2018) show that Southern Ocean deep-sea corals recorded lower pH during the Last Glacial Maximum (26,500–19,000 years ago), evidence that CO2 is stored in the deep ocean during glacial periods (Figure 2), while planktic δ11B (Shao et al., 2019) provides evidence of widespread outgassing of CO2 via the surface ocean during the last deglaciation.
|
|
Biomineralization
While many calcifiers have δ11B values close to borate at average seawater pH, some genera are notably offset from seawater values, providing insights into the mechanisms of biomineralization. For example, corals consistently record higher δ11B, and thus pH, than that of the water in which they grew, due to internal up-regulation of the calcifying fluid pH (McCulloch et al., 2012). This up-regulation process promotes carbonate precipitation by raising saturation state, and also helps concentrate carbon by creating a pronounced concentration gradient down which CO2 can diffuse from seawater (Allison et al., 2019). A deeper knowledge of these processes is vital for better understanding the resilience and response of marine calcifiers under rising anthropogenic CO2 emissions and acidifying ocean conditions.
Outlook
The strength of the boron isotope proxy is its foundation in inorganic acid-base and isotopic equilibria. The key uncertainty for CO2 system reconstruction on long timescales (>5 million years) is the boron isotopic composition of seawater, which has proved difficult to constrain and thus limits the accuracy of long-term pH and CO2 estimates (though relative changes can be reconstructed with more confidence). Despite recent analytical developments, boron isotope analyses also remain challenging. Gaining a better understanding of the CO2 system will require continued analytical improvements, understanding how boron is incorporated into marine carbonates, and knowledge of the constraints on long-term δ11B of seawater.