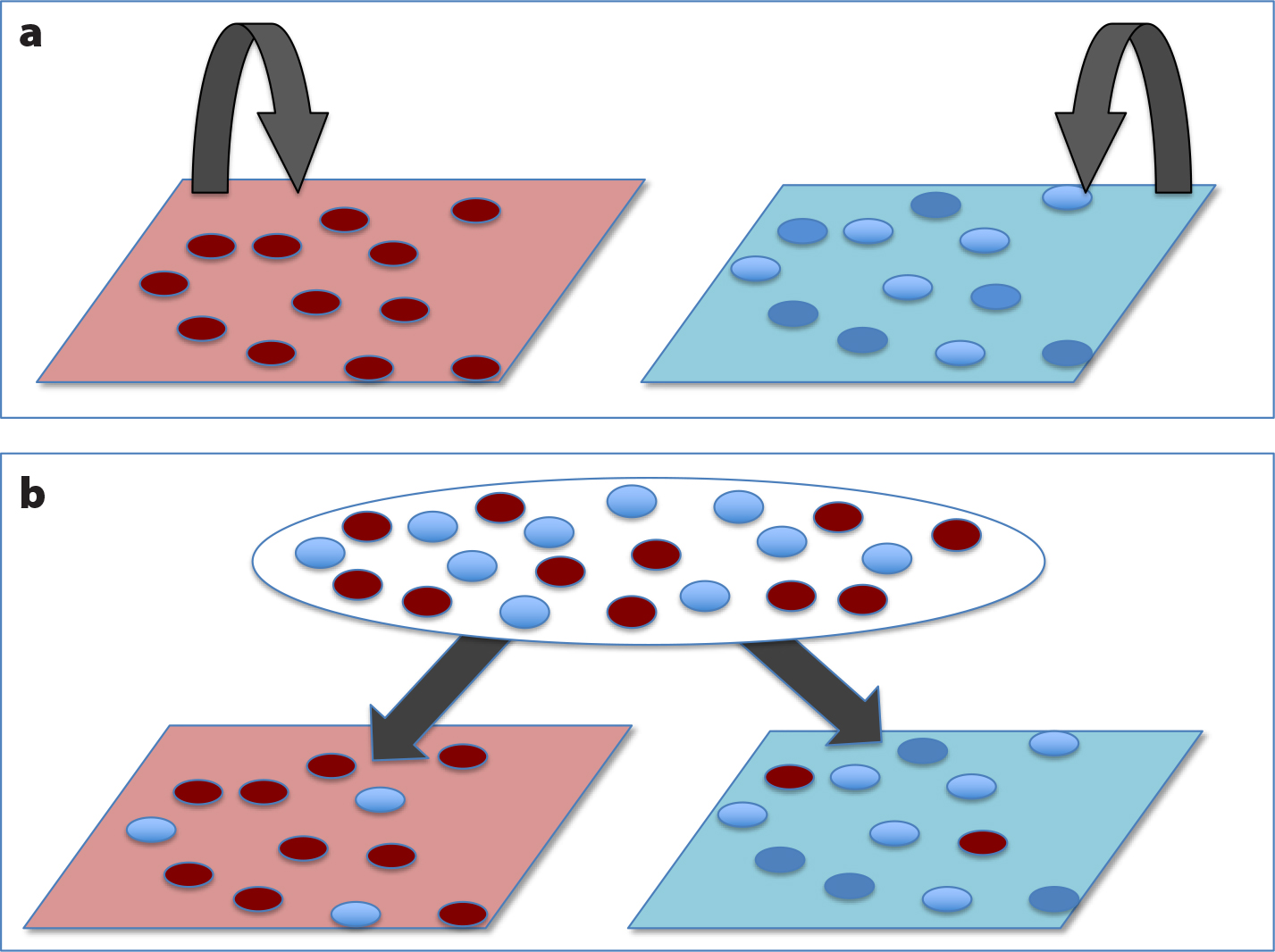
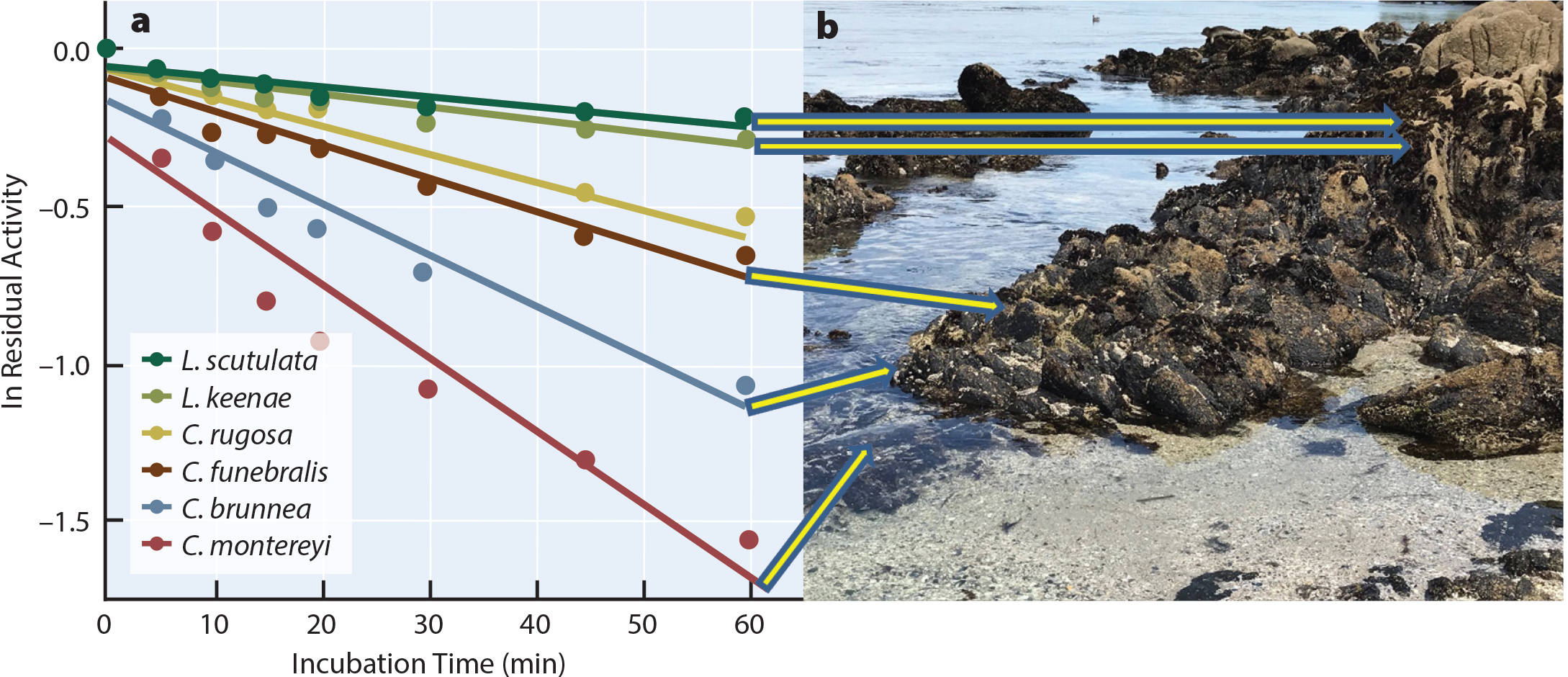
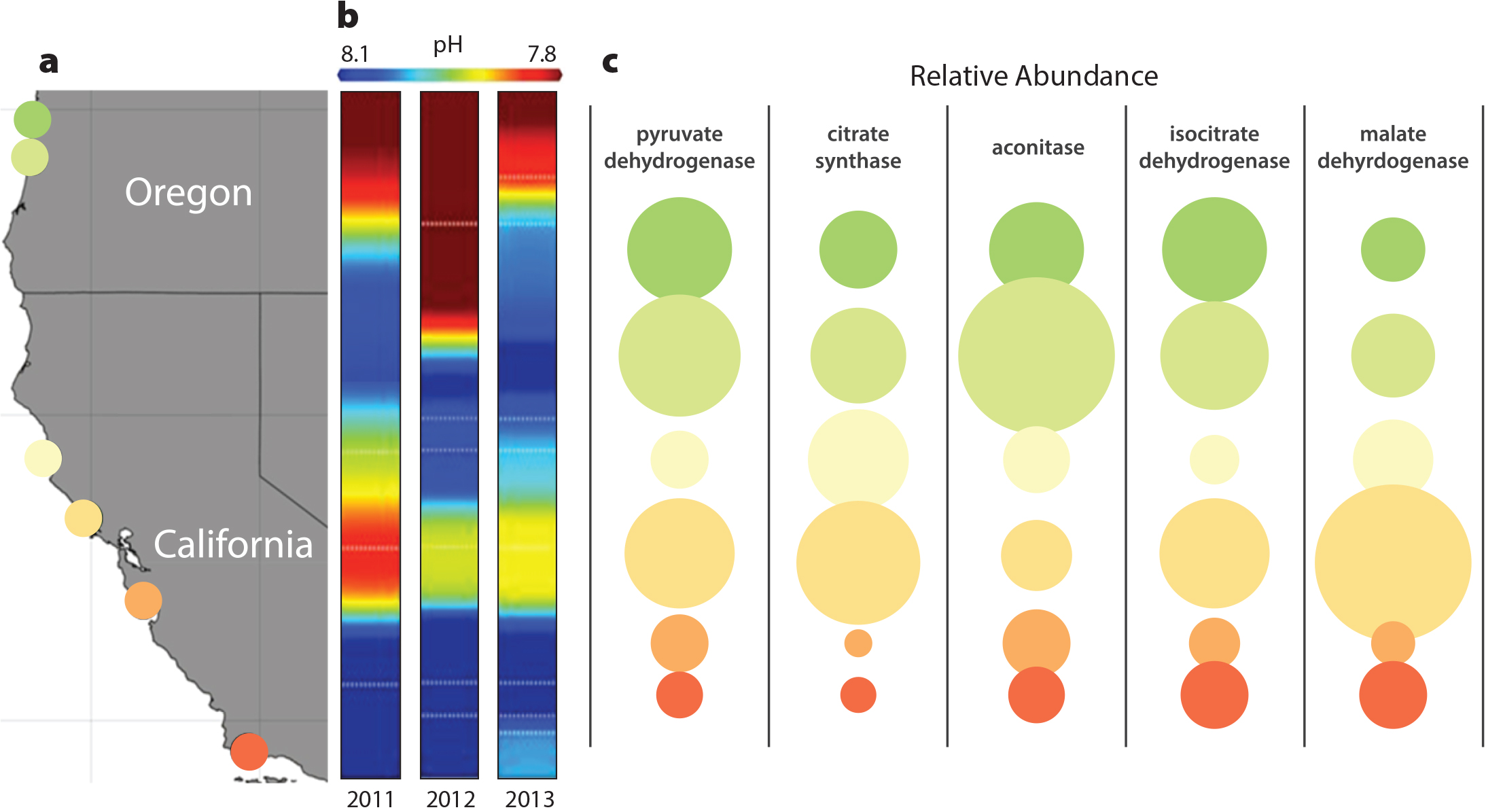
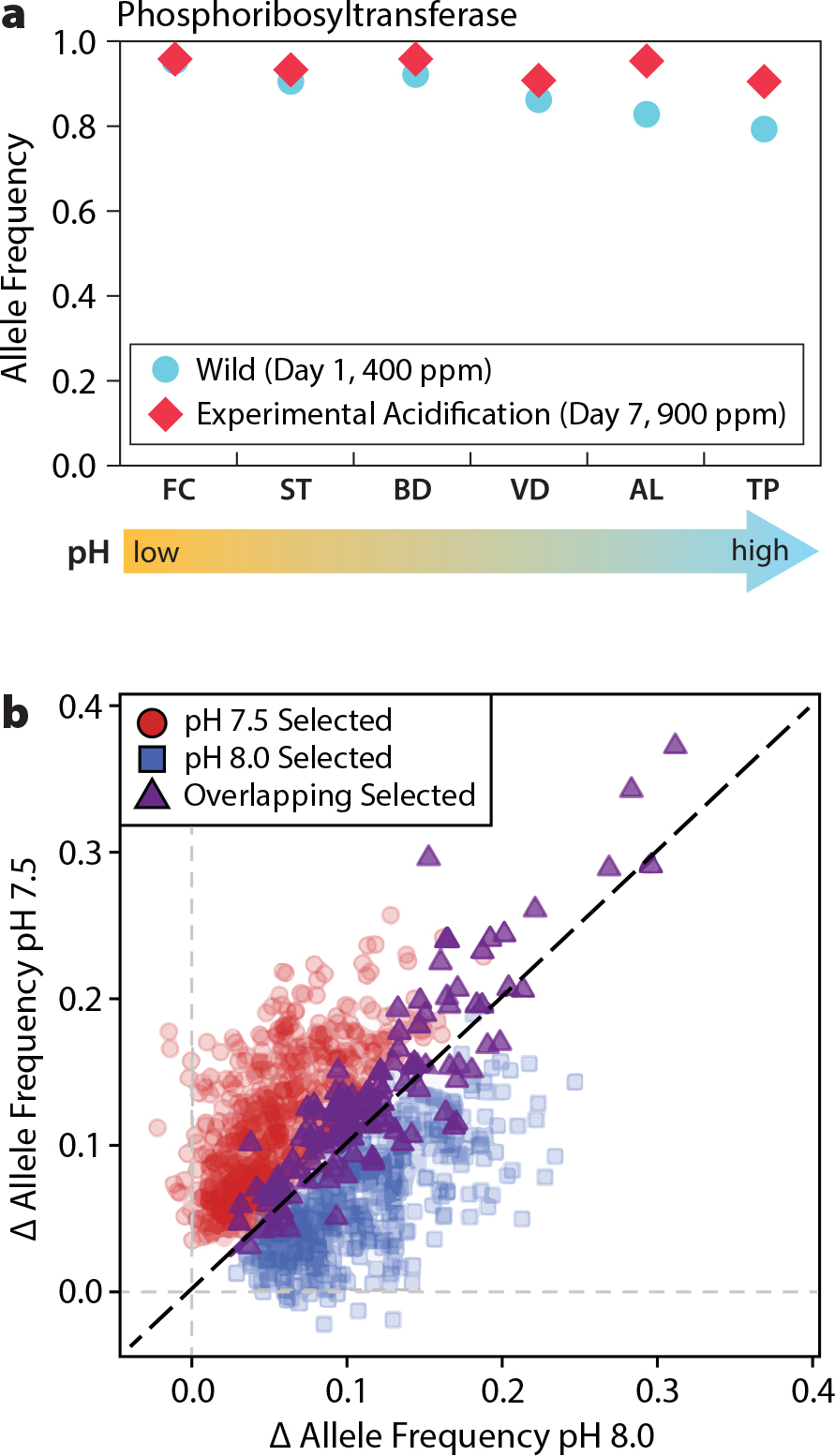
Barshis, D.J., E.E. Sotka, R.P. Kelly, A. Sivasundar, B.A. Menge, J.A. Barth, and S.R. Palumbi. 2011. Coastal upwelling is linked to temporal genetic variability in the acorn barnacle Balanus glandula. Marine Ecology Progress Series 439:139–150, https://doi.org/10.3354/meps09339.
Barth, J.M., P.R. Berg, P.R. Jonsson, S. Bonanomi, H. Corell, J. Hemmer-Hansen, K.S. Jakobsen, K. Johannesson, P.E. Jorde, H. Knutsen, and P.O. Moksnes. 2017. Genome architecture enables local adaptation of Atlantic cod despite high connectivity. Molecular Ecology 26(17):4,452–4,466, https://doi.org/10.1111/mec.14207.
Blanchette, C.A., C.M. Miner, P.T. Raimondi, D. Lohse, K.E. Heady, and B.R. Broitman. 2008. Biogeographical patterns of rocky intertidal communities along the Pacific coast of North America. Journal of Biogeography 35(9):1,593–1,607, https://doi.org/10.1111/j.1365-2699.2008.01913.x.
Block, B.A., I.D. Jonsen, S.J. Jorgensen, A.J. Winship, S.A. Shaffer, S.J. Bograd, E.L. Hazen, D.G. Foley, G.A. Breed, A.L. Harrison, and J.E. Ganong. 2011. Tracking apex marine predator movements in a dynamic ocean. Nature 475(7354):86–90, https://doi.org/10.1038/nature10082.
Bradbury, I.R., B. Laurel, P.V. Snelgrove, P. Bentzen, and S.E. Campana. 2008. Global patterns in marine dispersal estimates: The influence of geography, taxonomic category and life history. Proceedings of the Royal Society of London B 275(1644):1,803–1,809, https://doi.org/10.1098/rspb.2008.0216.
Brennan, R.S., A.D. Garrett, K. Huber, H. Hargarten, and M.H. Pespeni. 2019. Rare genetic variation is important for survival in extreme and moderate low pH conditions. bioRxiv, https://doi.org/10.1101/422261.
Burford, M.O. 2009. Demographic history, geographical distribution and reproductive isolation of distinct lineages of blue rockfish (Sebastes mystinus), a marine fish with a high dispersal potential. Journal of Evolutionary Biology 22(7):1,471–1,486, https://doi.org/10.1111/j.1420-9101.2009.01760.x.
Byrne, M., M. Lamare, D. Winter, S.A. Dworjanyn, and S. Uthicke. 2013. The stunting effect of a high CO2 ocean on calcification and development in sea urchin larvae, a synthesis from the tropics to the poles. Philosophical Transactions of the Royal Society B 368(1627):20120439, https://doi.org/10.1098/rstb.2012.0439.
Catullo, R.A., S. Ferrier, and A.A. Hoffmann. 2015. Extending spatial modelling of climate change responses beyond the realized niche: Estimating, and accommodating, physiological limits and adaptive evolution. Global Ecology and Biogeography 24(10):1,192–1,202, https://doi.org/10.1111/geb.12344.
Chan, F., J.A. Barth, C.A. Blanchette, R.H. Byrne, F. Chavez, O. Cheriton, R.A. Feely, G. Friederich, B. Gaylord, T. Gouhier, and S. Hacker. 2017. Persistent spatial structuring of coastal ocean acidification in the California Current System. Scientific Reports 7(1):2526, https://doi.org/10.1038/s41598-017-02777-y.
Deutsch, C., A. Ferrel, B. Seibel, H.O. Pörtner, and R.B. Huey. 2015. Climate change tightens a metabolic constraint on marine habitats. Science 348(6239):1,132–1,135, https://doi.org/10.1126/science.aaa1605.
De Wit, P., and S.R. Palumbi. 2013. Transcriptome-wide polymorphisms of red abalone (Haliotis rufescens) reveal patterns of gene flow and local adaptation. Molecular Ecology 22(11):2,884–2,897, https://doi.org/10.1111/mec.12081.
Di Santo, V., 2019. Ocean acidification and warming affect skeletal mineralization in a marine fish. Proceedings of the Royal Society B 286(1894), https://doi.org/10.1098/rspb.2018.2187.
Dong, Y.-W., and G.N. Somero. 2009. Temperature adaptation of cytosolic malate dehydrogenases of limpets (genus Lottia): Differences in stability and function due to minor changes in sequence correlate with biogeographic and vertical distributions. Journal of Experimental Biology 212:169–177, https://doi.org/10.1242/jeb.024505.
Dong, Y.-W., M.-L. Liao, X.-L. Meng, and G.N. Somero. 2018. Structural flexibility and protein adaptation to temperature: Molecular dynamics analysis of malate dehydrogenases of marine mollusks. Proceedings of the National Academy of Sciences of the United States of America 115(6):1,274–1,279, https://doi.org/10.1073/pnas.1718910115.
Evans, T.G., F. Chan, B.A. Menge, and G.E. Hofmann. 2013. Transcriptomic responses to ocean acidification in larval sea urchins from a naturally variable pH environment. Molecular Ecology 22(6):1,609–1,625, https://doi.org/10.1111/mec.12188.
Evans, T.G., J.L. Padilla-Gamiño, M.W. Kelly, M.H. Pespeni, F. Chan, B.A. Menge, B. Gaylord, T.M. Hill, A.D. Russell, S.R. Palumbi, and others. 2015. Ocean acidification research in the ‘post-genomic’ era: Roadmaps from the purple sea urchin Strongylocentrotus purpuratus. Comparative Biochemistry and Physiology Part A 185:33–42, https://doi.org/10.1016/j.cbpa.2015.03.007.
Evans, T.G., M.H. Pespeni, G.E. Hofmann, S.R. Palumbi, and E. Sanford, 2017. Transcriptomic responses to seawater acidification among sea urchin populations inhabiting a natural pH mosaic. Molecular Ecology 26(8):2,257–2,275, https://doi.org/10.1111/mec.14038.
Feely, R.A., C.L. Sabine, J.M. Hernandez-Ayon, D. Ianson, and B. Hales. 2008. Evidence for upwelling of corrosive “acidified” water onto the continental shelf. Science 320(5882):1,490–1,492, https://doi.org/10.1126/science.1155676.
Fields, P.A., Y.-W Dong, X. Meng, and G.N. Somero. 2015. Adaptations of protein structure and function to temperature: There is more than one way to “skin the cat.” Journal of Experimental Biology 218:1,801–1,811, https://doi.org/10.1242/jeb.114298.
Galindo, H.M., A.S. Pfeiffer-Herbert, M.A. McManus, Y. Chao, F. Chai, and S.R. Palumbi. 2010. Seascape genetics along a steep cline: Using genetic patterns to test predictions of marine larval dispersal. Molecular Ecology 19(17):3,692–3,707, https://doi.org/10.1111/j.1365-294X.2010.04694.x.
Hofmann, G.E., J.E. Smith, K.S. Johnson, U. Send, L.A. Levin, F. Micheli, A. Paytan, N.N. Price, B. Peterson, Y. Takeshita, and P.G. Matson. 2011. High-frequency dynamics of ocean pH: A multi-ecosystem comparison. PLOS ONE 6(12): e28983, https://doi.org/10.1371/journal.pone.0028983.
Jackson, J.B.C. 1974. Biogeographic consequences of eurytopy and stenotopy among marine bivalves and their evolutionary significance. The American Naturalist 108(962):541–560, https://doi.org/10.1086/282933.
Johansson, M.L., F. Alberto, D.C. Reed, P.T. Raimondi, N.C. Coelho, M.A. Young, P.T. Drake, C.A. Edwards, K. Cavanaugh, J. Assis, and L.B. Ladah. 2015. Seascape drivers of Macrocystis pyrifera population genetic structure in the northeast Pacific. Molecular Ecology 24(19):4,866–4,885, https://doi.org/10.1111/mec.13371.
Keeling, R.F. 2008. Recording Earth’s vital signs. Science 319(5871):1,771–1,772, https://doi.org/10.1126/science.1156761.
Kelly, M.W., and G.E. Hofmann. 2013. Adaptation and the physiology of ocean acidification. Functional Ecology 27(4):980–990, https://doi.org/10.1111/j.1365-2435.2012.02061.x.
Kelly, M.W., J.L. Padilla-Gamiño, and G.E. Hofmann. 2013. Natural variation and the capacity to adapt to ocean acidification in the keystone sea urchin Strongylocentrotus purpuratus. Global Change Biology 19(8):2,536–2,546, https://doi.org/10.1111/gcb.12251.
Kelly, R.P., and S.R. Palumbi. 2010. Genetic structure among 50 species of the northeastern Pacific rocky intertidal community. PLOS ONE 5(1):e8594, https://doi.org/10.1371/journal.pone.0008594.
Kroeker, K.J., R.L. Kordas, R.N. Crim, and G.G. Singh. 2010. Meta-analysis reveals negative yet variable effects of ocean acidification on marine organisms. Ecology Letters 13(11):1,419–1,434, https://doi.org/10.1111/j.1461-0248.2010.01518.x.
Levene, H. 1953. Genetic equilibrium when more than one ecological niche is available. The American Naturalist 87(836):331–333, https://doi.org/10.1086/281792.
Liao, M.-L., G.N. Somero, and Y.-W. Dong. 2019. Comparing mutagenesis and simulations as tools for identifying functionally important sequence changes for protein thermal adaptation. Proceedings of the National Academy of Sciences of the United States of America 116(2):679–688, https://doi.org/10.1073/pnas.1817455116.
Lloyd, M.M., A.D. Makukhov, and M.H. Pespeni. 2016. Loss of genetic diversity as a consequence of selection in response to high pCO2. Evolutionary Applications 9(9):1,124–1,132, https://doi.org/10.1111/eva.12404.
Lockwood, B.L., and G.N. Somero. 2011. Invasive and native blue mussels (genus Mytilus) on the California coast: The role of physiology in a biological invasion. Journal of Experimental Marine Biology and Ecology 400 (2011):167–174, https://doi.org/10.1016/j.jembe.2011.02.022.
Lockwood, B.L., and G.N. Somero. 2012. Functional determinants of temperature adaptation in enzymes of cold- versus warm-adapted mussels (genus Mytilus). Molecular Biology and Evolution 29(10):3,061–3,070, https://doi.org/10.1093/molbev/mss111.
Low, N.H., and F. Micheli. 2018. Lethal and functional thresholds of hypoxia in two key benthic grazers. Marine Ecology Progress Series 594:165–173, https://doi.org/10.3354/meps12558.
Martz, T.R., J.G. Connery, and K.S. Johnson. 2010. Testing the Honeywell Durafet® for seawater pH applications. Limnology and Oceanography Methods 8:172–184, https://doi.org/10.4319/lom.2010.8.172.
Norberg, J., M.C. Urban, M. Vellend, C.A. Klausmeier, and N. Loeuille. 2012. Eco-evolutionary responses of biodiversity to climate change. Nature Climate Change 2(10):747–751, https://doi.org/10.1038/nclimate1588.
Pacella, S.R., C.A. Brown, G.G. Waldbusser, R.G. Labiosa, and B. Hales. 2018. Seagrass habitat metabolism increases short-term extremes and long-term offset of CO2 under future ocean acidification. Proceedings of the National Academy of Sciences of the United States of America 115(15):3,870–3,875, https://doi.org/10.1073/pnas.1703445115.
Pan, T.C.F., S.L. Applebaum, and D.T. Manahan. 2015. Experimental ocean acidification alters the allocation of metabolic energy. Proceedings of the National Academy of Sciences of the United States of America 112(15):4,696–4,701, https://doi.org/10.1073/pnas.1416967112.
Pearse, J.S. 2006. Ecological role of purple sea urchins. Science 314(5801):940–941, https://doi.org/10.1126/science.1131888.
Pespeni, M.H., T.A. Oliver, M.K. Manier, and S.R. Palumbi. 2010. Restriction Site Tiling Analysis: Accurate discovery and quantitative genotyping of genome-wide polymorphisms using nucleotide arrays. Genome Biology 11:R44, https://doi.org/10.1186/gb-2010-11-4-r44.
Pespeni, M.H., D.A. Garfield, M.K. Manier, and S.R. Palumbi. 2012. Genome-wide polymorphisms show unexpected targets of natural selection. Proceedings of the Royal Society B 279(1732), https://doi.org/10.1098/rspb.2011.1823.
Pespeni, M.H., and S.R. Palumbi. 2013. Signals of selection in outlier loci in a widely dispersing species across an environmental mosaic. Molecular Ecology 22(13):3,580–3,597, https://doi.org/10.1111/mec.12337.
Pespeni, M.H., E. Sanford, B. Gaylord, T.M Hill, J.D. Hosfelt, H.K. Jaris, M. LaVigne, E.A. Lenz, A.D. Russell, M.K. Young, and S.R. Palumbi. 2013a. Evolutionary change during experimental ocean acidification. Proceedings of the National Academy of Sciences of the United States of America 110(17):6,937–6,942, https://doi.org/10.1073/pnas.1220673110.
Pespeni, M.H., B.T. Barney, and S.R. Palumbi. 2013b. Differences in the regulation of growth and biomineralization genes revealed through long-term common-garden acclimation and experimental genomics in the purple sea urchin. Evolution 67(7):1,901–1,914, https://doi.org/10.1111/evo.12036.
Pespeni, M.H., F. Chan, B.A. Menge, and S.R. Palumbi. 2013c. Signs of adaptation to local pH conditions across an environmental mosaic in the California Current ecosystem. Integrative and Comparative Biology 53(5):857–870, https://doi.org/10.1093/icb/ict094.
Pinsky, M.L., B. Worm, M.J. Fogarty, J.L. Sarmiento, and S.A. Levin. 2013. Marine taxa track local climate velocities. Science 341(6151):1,239–1,242, https://doi.org/10.1126/science.1239352.
Richardson, J.L., M.C. Urban, D.I. Bolnick, and D.K. Skelly. 2014. Microgeographic adaptation and the spatial scale of evolution. Trends in Ecology & Evolution, 29(3):165–176, https://doi.org/10.1016/j.tree.2014.01.002.
Roy, K., D. Jablonski, J.W. Valentine, and G. Rosenberg. 1998. Marine latitudinal diversity gradients: Tests of causal hypotheses. Proceedings of the National Academy of Sciences of the United States of America 95(7):3,699–3,702, https://doi.org/10.1073/pnas.95.7.3699.
Sabine, C.L., R.A. Feely, N. Gruber, R.M. Key, K. Lee, J.L. Bullister, R. Wanninkhof, C.S.L. Wong, D.W. Wallace, B. Tilbrook, and F.J. Millero. 2004. The oceanic sink for anthropogenic CO2. Science 305(5682):367–371, https://doi.org/10.1126/science.1097403.
Sanford, E., and M.W. Kelly. 2011. Local adaptation in marine invertebrates. Annual Review of Marine Science 3:509–535, https://doi.org/10.1146/annurev-marine-120709-142756.
Shum, P., B.T. Barney, J. O’Leary, and S.R. Palumbi. In press. Cobble community DNA as a tool to monitor patterns of biodiversity within kelp forest ecosystems. Molecular Ecology Resources, https://doi.org/10.5061/dryad.hq229kc.
Sivasundar, A., and S.R. Palumbi. 2010. Life history, ecology and the biogeography of strong genetic breaks among 15 species of Pacific rockfish, Sebastes. Marine Biology 157(7):1,433–1,452, https://doi.org/10.1007/s00227-010-1419-3.
Somero, G.N., J.M. Beers, F. Chan, T.M Hill, T. Klinger, and S.Y. Litvin. 2016. What changes in the carbonate system, oxygen, and temperature portend for the Northeastern Pacific Ocean: A physiological perspective. BioScience 66(1):14–26, https://doi.org/10.1093/biosci/biv162.
Somero, G.N., B.L. Lockwood, and L. Tomanek. 2017. Biochemical Adaptation: Response to Environmental Challenges from Life’s Origins to the Anthropocene. Sinauer Associates, Sunderland, MA, 572 pp.
Sotka, E.E., J.P. Wares, J.A. Barth, R.K. Grosberg, and S.R. Palumbi. 2004. Strong genetic clines and geographical variation in gene flow in the rocky intertidal barnacle Balanus glandula. Molecular Ecology 13(8):2,143–2,156.
Stumpp, M., M.Y. Hu, F. Melzner, M.A. Gutowska, N. Dorey, N. Himmerkus, W.C. Holtmann, S.T. Dupont, M.C. Thorndyke, and M. Bleich. 2012. Acidified seawater impacts sea urchin larvae pH regulatory systems relevant for calcification. Proceedings of the National Academy of Sciences of the United States of America 109(44):18,192–18,197, https://doi.org/10.1073/pnas.1209174109.
van Oppen, M.J., J.K. Oliver, H.M. Putnam, and R.D. Gates. 2015. Building coral reef resilience through assisted evolution. Proceedings of the National Academy of Sciences of the United States of America 112(8):2,307–2,313, https://doi.org/10.1073/pnas.1422301112.