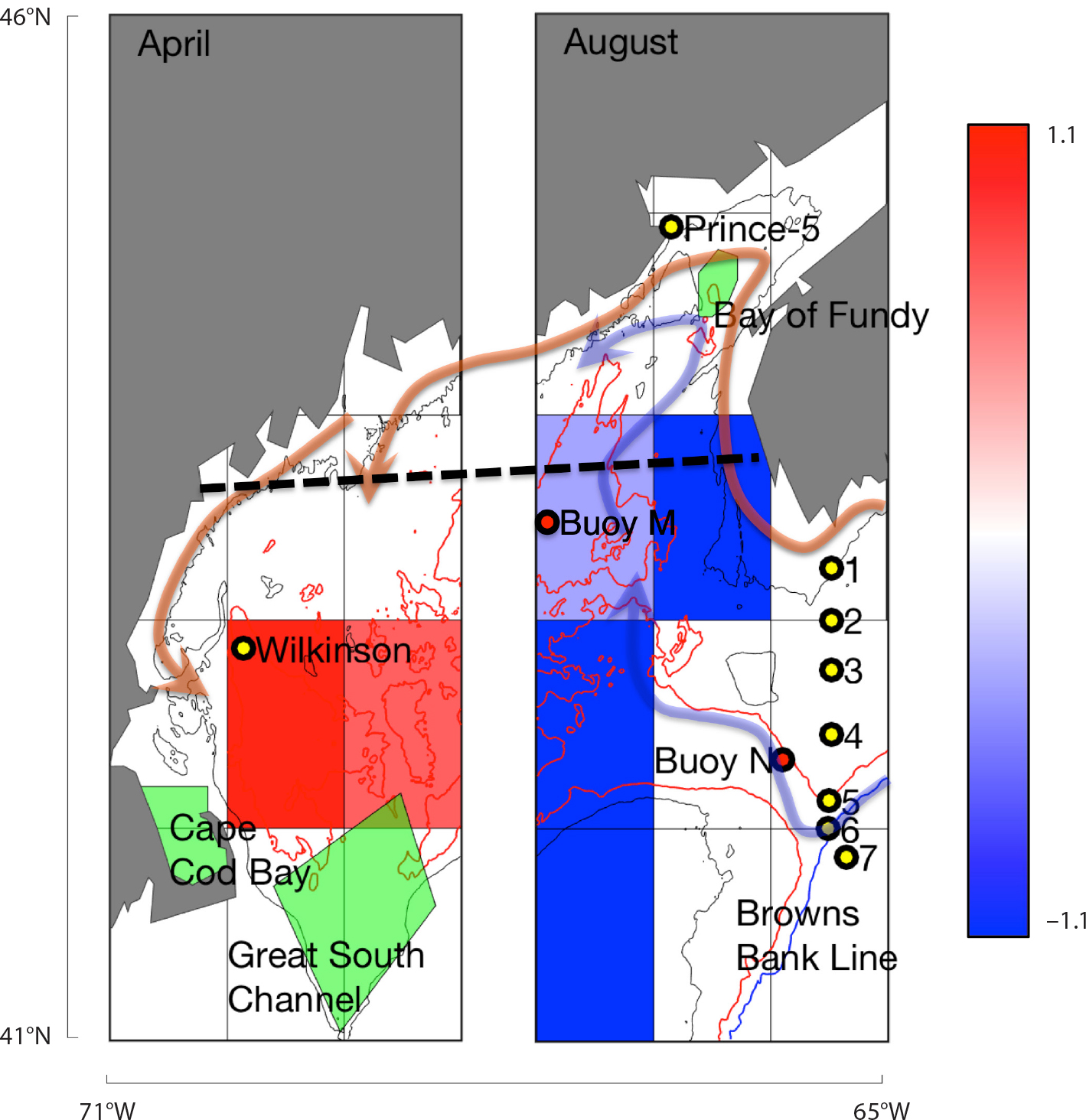
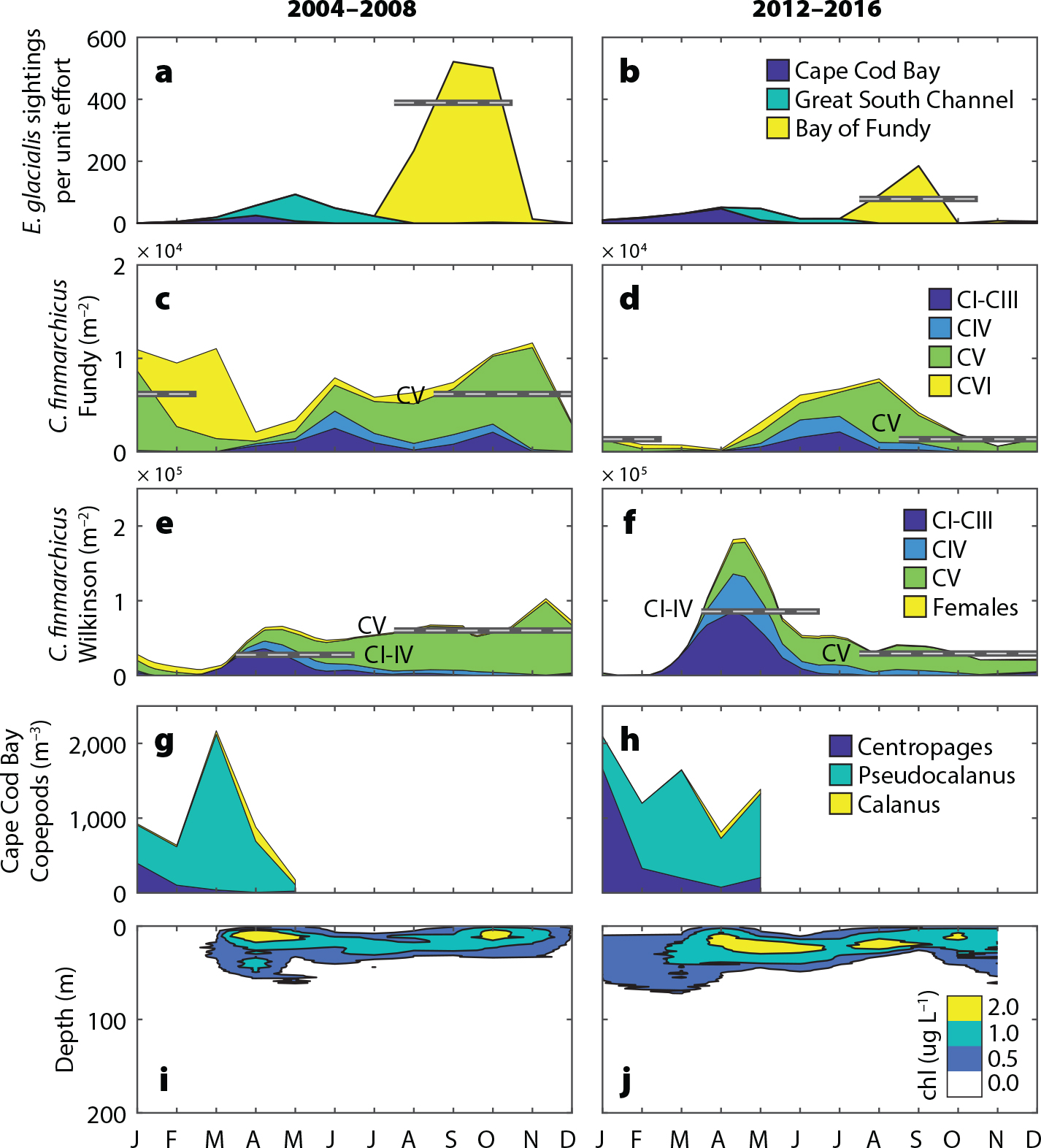
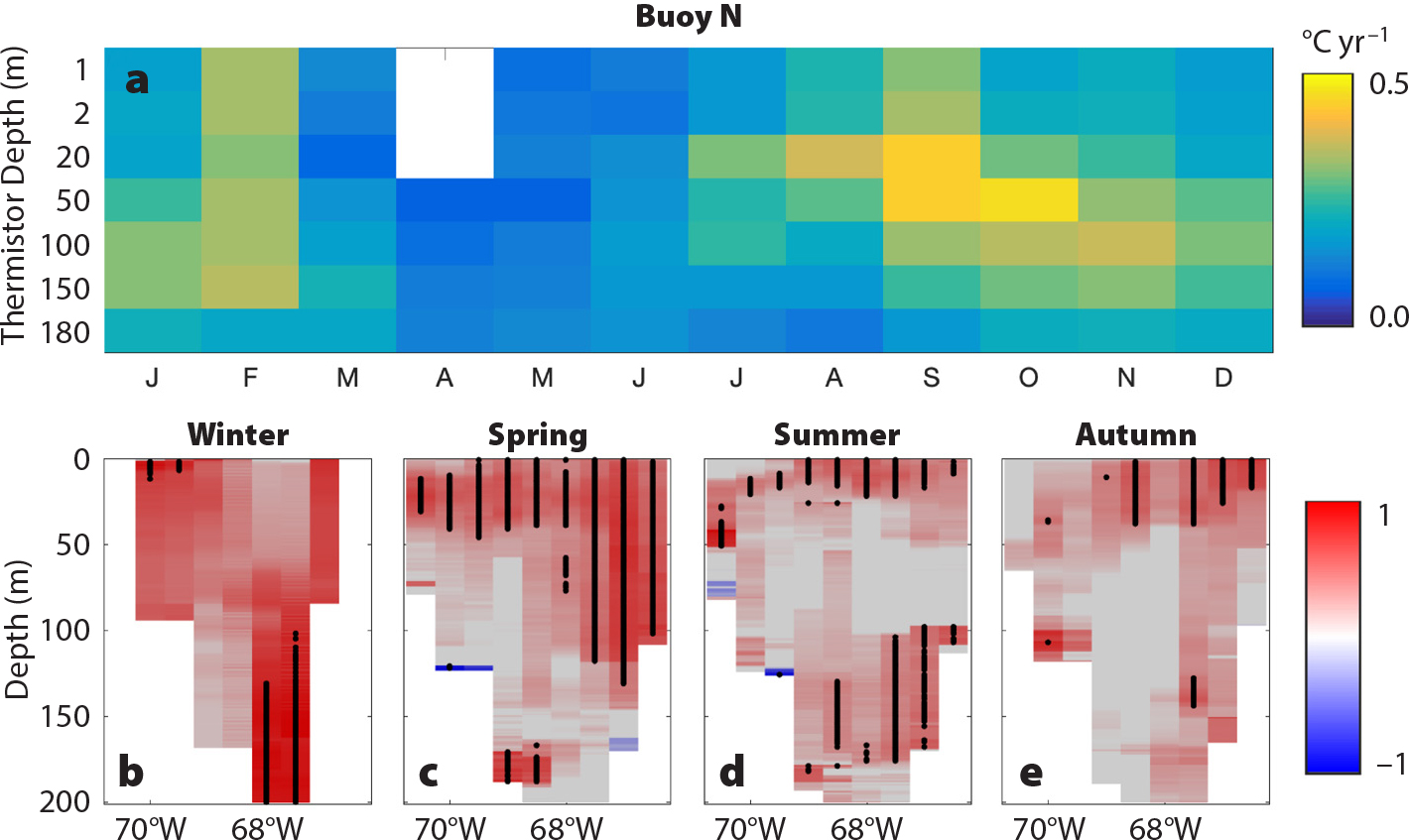
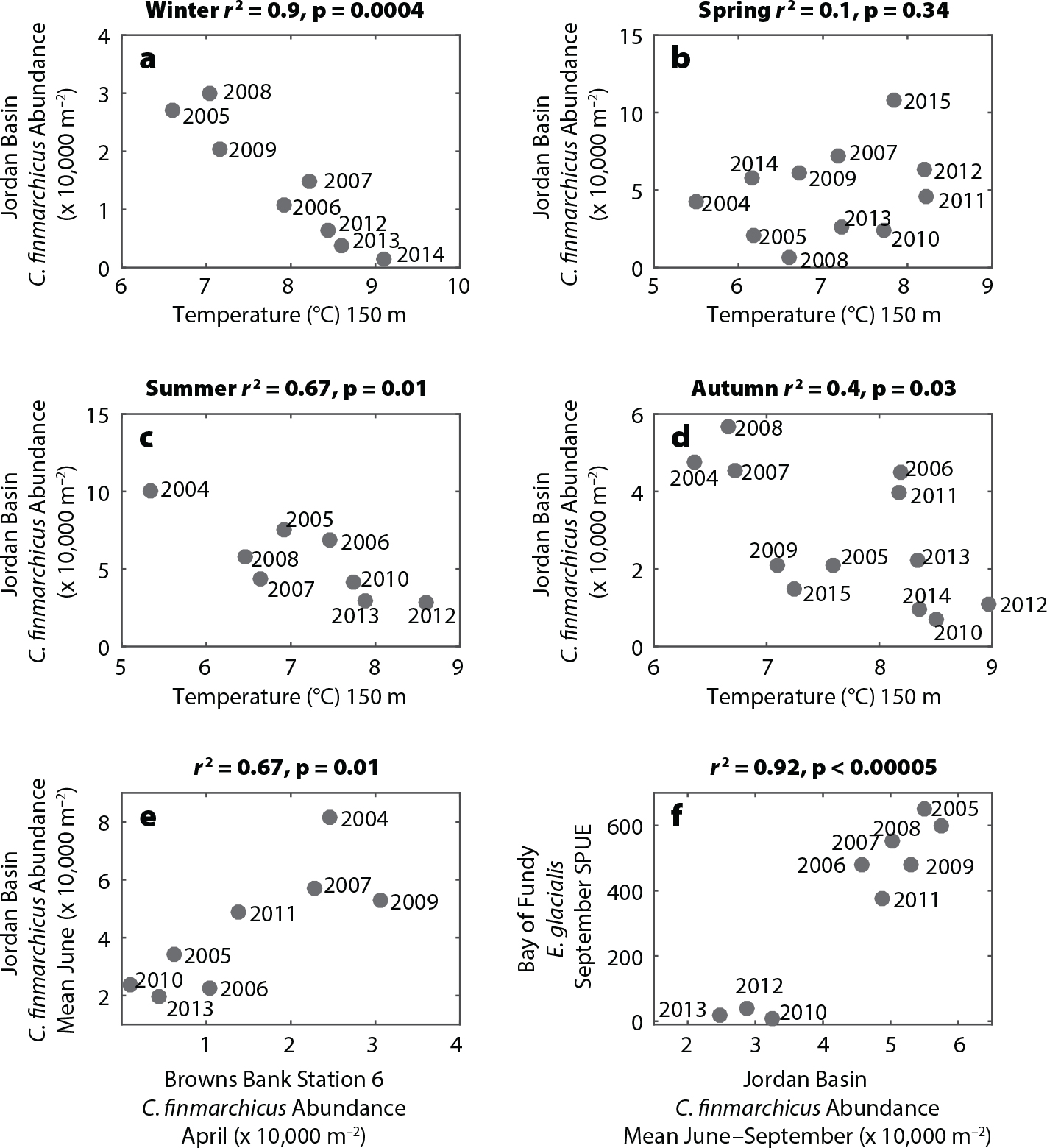
Beardsley, R.C., A.W. Epstein, C.S. Chen, K.F. Wishner, M.C. Macaulay, and R.D. Kenney. 1996. Spatial variability in zooplankton abundance near feeding right whales in the Great South Channel. Deep-Sea Research Part II 43(7–8):1,601–1,625, https://doi.org/10.1016/S0967-0645(96)00050-1.
Caesar, L., S. Rahmstorf, A. Robinson, G. Feulner, and V. Saba. 2018. Observed fingerprint of a weakening Atlantic Ocean overturning circulation. Nature 556(7700):191–196, https://doi.org/10.1038/s41586-018-0006-5.
Chen, I.C., J.K. Hill, R. Ohlemüller, D.B. Roy, and C.D. Thomas. 2011. Rapid range shifts of species associated with high levels of climate warming. Science 333(6045):1,024–1,026, https://doi.org/10.1126/science.1206432.
Cheung, W.W., V.W. Lam, J.L. Sarmiento, K. Kearney, R. Watson, and D. Pauly. 2009. Projecting global marine biodiversity impacts under climate change scenarios. Fish and Fisheries 10(3):235–251, https://doi.org/10.1111/j.1467-2979.2008.00315.x.
Corkeron, P., P. Hamilton, J. Bannister, P. Best, C. Charlton, K.R. Groch, K. Findlay, V. Rowntree, E. Vermeulen, and R.M. Pace III. 2018. The recovery of North Atlantic right whales, Eubalaena glacialis, has been constrained by human-caused mortality. Royal Society Open Science 5(11):180892, https://doi.org/10.1098/rsos.180892.
Daoust, P.-Y., E.L. Couture, T. Wimmer, and L. Bourque. 2017. Incident Report: North Atlantic Right Whale Mortality Event in the Gulf of St. Lawrence, 2017. Collaborative Report produced by Canadian Wildlife Health Cooperative, Marine Animal Response Society, Fisheries and Oceans Canada, 224 pp.
Davies, K.T., and S.W. Brillant. 2019. Mass human-caused mortality spurs federal action to protect endangered North Atlantic right whales in Canada. Marine Policy 104:157–162, https://doi.org/10.1016/j.marpol.2019.02.019.
Davis, G.E., M.F. Baumgartner, J.M. Bonnell, J. Bell, C. Berchok, J.B. Thornton, S. Brault, G. Buchanan, R.A. Charif, D. Cholewiak, and C.W. Clark. 2017. Long-term passive acoustic recordings track the changing distribution of North Atlantic right whales (Eubalaena glacialis) from 2004 to 2014. Scientific Reports 7(1):13460, https://doi.org/10.1038/s41598-017-13359-3.
Di Lorenzo, E., and N. Mantua. 2016. Multi-year persistence of the 2014/15 North Pacific marine heatwave. Nature Climate Change 6(11):1,042–1,047, https://doi.org/10.1038/nclimate3082.
Durbin, E.G., R.G. Campbell, M.C. Casas, M.D. Ohman, B. Niehoff, J. Runge, and M. Wagner. 2003. Interannual variation in phytoplankton blooms and zooplankton productivity and abundance in the Gulf of Maine during winter. Marine Ecology Progress Series 254:81–100, https://doi.org/10.3354/meps254081.
Ganley, L.C., S. Brault, and C.A. Mayo. 2019. What we see is not what there is: Estimating North Atlantic right whale Eubalaena glacialis local abundance. Endangered Species Research 38:101–113, https://doi.org/10.3354/esr00938.
Gibbons, J.D., and S. Chakraborti. 2011. Nonparametric Statistical Inference, 5th ed. Chapman & Hall/CRC Press, Taylor & Francis Group, Boca Raton, FL, 650 pp.
Greene, C.H., A.J. Pershing, B.C. Monger, M.C. Benfield, E.G. Durbin, and M.C. Casas. 2004. Supply-side ecology and the response of zooplankton to climate-driven changes in the North Atlantic Ocean. Oceanography 17(3):60–71, https://doi.org/10.5670/oceanog.2004.31.
Head, E.J.H., L.R. Harris, and B. Petrie. 1999. Distribution of Calanus spp. on and around the Nova Scotia shelf in April: Evidence for an offshore source of Calanus finmarchicus to the mid- and western regions. Canadian Journal of Fisheries and Aquatic Sciences 56:2,463–2,476, https://doi.org/10.1139/f99-193.
Jewett, L., and A. Romanou. 2017. Ocean acidification and other ocean changes. Pp. 364–392 in Climate Science Special Report: Fourth National Climate Assessment, Volume I. D.J. Wuebbles, D.W. Fahey, K.A. Hibbard, D.J. Dokken, B.C. Stewart, and T.K. Maycock, eds, US Global Change Research Program, Washington, DC.
Ji, R., Z. Feng, B.T. Jones, C. Thompson, C. Chen, N.R. Record, and J.A. Runge. 2017. Coastal amplification of supply and transport (CAST): A new hypothesis about the persistence of Calanus finmarchicus in the Gulf of Maine. ICES Journal of Marine Science 74(7):1,865–1,874, https://doi.org/10.1093/icesjms/fsw253.
Kenney, R.D., M.A.M. Hyman, R.E. Owen, G.P. Scott, and H.E. Winn. 1986. Estimation of prey densities required by western North Atlantic right whales. Marine Mammal Science 2:1–13, https://doi.org/10.1111/j.1748-7692.1986.tb00024.x.
Kraus, S.D., R.D. Kenney, C.A. Mayo, W.A. McLellan, M.J. Moore, and D.P. Nowacek. 2016. Recent scientific publications cast doubt on North Atlantic right whale future. Frontiers in Marine Science 3:137, https://doi.org/10.3389/fmars.2016.00137.
Leiter, S.M., K.M. Stone, J.L. Thompson, C.M. Accardo, B.C. Wikgren, M.A. Zani, T.V.N. Cole, R.D. Kenney, C.A. Mayo, and S.D. Kraus. 2017. North Atlantic right whale Eubalaena glacialis occurrence in offshore wind energy areas near Massachusetts and Rhode Island, USA. Endangered Species Research 34:45–59, https://doi.org/10.3354/esr00827.
Mayo, C.A., and M.K. Marx. 1990. Surface foraging behaviour of the North Atlantic right whale, Eubalaena glacialis, and associated zooplankton characteristics. Canadian Journal of Zoology 68(10):2,214–2,220, https://doi.org/10.1139/z90-308.
Melle, W., J.A. Runge, E. Head, S. Plourde, C. Castellani, P. Licandro, J. Pierson, S. Jonasdottir, C. Johnson, C. Broms, and others. 2014. The North Atlantic Ocean as habitat for Calanus finmarchicus: Environmental factors and life history traits. Progress in Oceanography 129(B):244–284, https://doi.org/10.1016/j.pocean.2014.04.026.
Meyer-Gutbrod, E.L., and C.H. Greene. 2018. Uncertain recovery of the North Atlantic right whale in a changing ocean. Global Change Biology 24:455–464, https://doi.org/10.1111/gcb.13929.
Meyer-Gutbrod, E.L., C.H. Greene, and K.T.A. Davies. 2018. Marine species range shifts necessitate advanced policy planning: The case of the North Atlantic right whale. Oceanography 31(2):19–23, https://doi.org/10.5670/oceanog.2018.209.
Mills, K.E., A.J. Pershing, C.J. Brown, Y. Chen, F.S. Chiang, D.S. Holland, S. Lehuta, J.A. Nye, J.C. Sun, A.C. Thomas, and R.A. Wahle. 2013. Fisheries management in a changing climate: Lessons from the 2012 ocean heat wave in the Northwest Atlantic. Oceanography 26(2):191–195, https://doi.org/10.5670/oceanog.2013.27.
Murison, L.D., and D.E. Gaskin. 1989. The distribution of right whales and zooplankton in the Bay of Fundy, Canada. Canadian Journal of Zoology 67(6):1,411–1,420, https://doi.org/10.1139/z89-200.
Neckles, H.A. 2015. Loss of eelgrass in Casco Bay, Maine, linked to green crab disturbance. Northeastern Naturalist 22(3):478–500, https://doi.org/10.1656/045.022.0305.
Pace, R.M. III, P.J. Corkeron, and S.D. Kraus. 2017. State-space mark-recapture estimates reveal a recent decline in abundance of North Atlantic right whales. Ecology and Evolution 7(21):8,730–8,741, https://doi.org/10.1002/ece3.3406.
Pacifici, M., P. Visconti, S.H. Butchart, J.E. Watson, F.M. Cassola, and C. Rondinini. 2017. Species’ traits influenced their response to recent climate change. Nature Climate Change 7(3):205, https://doi.org/10.1038/nclimate3223.
Pendleton, D.E., A.J. Pershing, M.W. Brown, C.A. Mayo, R.D. Kenney, N.R. Record, and T.V.N. Cole. 2009. Regional-scale mean copepod concentration indicates relative abundance of North Atlantic right whales. Marine Ecology Progress Series 378:211–225, https://doi.org/10.3354/meps07832.
Pendleton, D.E., P.J. Sullivan, M.W. Brown, T.V. Cole, C.P. Good, C.A. Mayo, B.C. Monger, S. Phillips, N.R. Record, and A.J. Pershing. 2012. Weekly predictions of North Atlantic right whale Eubalaena glacialis habitat reveal influence of prey abundance and seasonality of habitat preferences. Endangered Species Research 18(2):147–161, https://doi.org/10.3354/esr00433.
Perry, A.L., P.J. Low, J.R. Ellis, and J.D. Reynolds. 2005. Climate change and distribution shifts in marine fishes. Science 308(5730):1,912–1,915, https://doi.org/10.1126/science.1111322.
Pershing, A.J., M.A. Alexander, C.M. Hernandez, L.A. Kerr, A. Le Bris, K.E. Mills, J.A. Nye, N.R. Record, H.A. Scannell, J.D. Scott, and G.D. Sherwood. 2015. Slow adaptation in the face of rapid warming leads to collapse of the Gulf of Maine cod fishery. Science 350(6262):809–812, https://doi.org/10.1126/science.aac9819.
Pershing, A.J., K.E. Mills, A.M. Dayton, B.S. Franklin, and B.T. Kennedy. 2018. Evidence for adaptation from the 2016 marine heatwave in the Northwest Atlantic Ocean. Oceanography 31(2):152–161, https://doi.org/10.5670/oceanog.2018.213.
Pettis, H., and P. Hamilton. 2016. North Atlantic Right Whale Consortium 2016 annual report card. North Atlantic Right Whale Consortium, Boston, MA, https://www.narwc.org/report-cards.html.
Pettis, H.M., R.M. Pace III, and P.K. Hamilton. 2018. North Atlantic Right Whale Consortium 2018 annual report card. North Atlantic Right Whale Consortium, Boston, MA, https://www.narwc.org/report-cards.html.
Pinsky, M.L., B. Worm, M.J. Fogarty, J.L. Sarmiento, and S.A. Levin. 2013. Marine taxa track local climate velocities. Science 341(6151):1,239–1,242, https://doi.org/10.1126/science.1239352.
Rahmstorf, S., J.E. Box, G. Feulner, M.E. Mann, A. Robinson, S. Rutherford, and E.J. Schaffernicht. 2015. Exceptional twentieth-century slowdown in Atlantic Ocean overturning circulation. Nature Climate Change 5(5):475, https://doi.org/10.1038/nclimate2554.
Rolland, R.M., R.S. Schick, H.M. Pettis, A.R. Knowlton, P.K. Hamilton, J.S. Clark, and S.D. Kraus. 2016. Health of North Atlantic right whales, Eubalaena glacialis, over three decades: From individual health to demographic and population health trends. Marine Ecology Progress Series 542:265–282, https://doi.org/10.3354/meps11547.
Runge, J.A., R. Ji, C.R.S. Thompson, N.R. Record, C. Chen, D.C. Vandemark, J.E. Salisbury, and F. Maps. 2015. Persistence of Calanus finmarchicus in the western Gulf of Maine during recent extreme warming. Journal of Plankton Research 37(1):221–232, https://doi.org/10.1093/plankt/fbu098.
Saba, V.S., S.M. Griffies, W.G. Anderson, M. Winton, M.A. Alexander, T.L. Delworth, J.A. Hare, M.J. Harrison, A. Rosati, G.A. Vecchi, and R. Zhang. 2016. Enhanced warming of the Northwest Atlantic Ocean under climate change. Journal of Geophysical Research 121(1):118–132, https://doi.org/10.1002/2015JC011346.
Sherwood, O.A., M.F. Lehmann, C.J. Schubert, D.B. Scott, and M.D. McCarthy. 2011. Nutrient regime shift in the western North Atlantic indicated by compound-specific δ15N of deep-sea gorgonian corals. Proceedings of the National Academy of Sciences of the United States of America 108(3):1,011–1,015, https://doi.org/10.1073/pnas.1004904108.
Speirs, D.C., W.S. Gurney, M.R. Heath, W. Horbelt, S.N. Wood, and B.A. DeCuevas. 2006. Ocean-scale modelling of the distribution, abundance, and seasonal dynamics of the copepod Calanus finmarchicus. Marine Ecology Progress Series 313:173–192, https://doi.org/10.3354/meps313173.
Stokstad, E. 2017. Surge in right whale deaths raises alarms. Science 357(6353):740–741, https://doi.org/10.1126/science.357.6353.740.
Thomas, A.C., A.J. Pershing, K.D. Friedland, J.A. Nye, K.E. Mills, M.A. Alexander, N.R. Record, R. Weatherbee, and M.E. Henderson. 2017. Seasonal trends and phenology shifts in sea surface temperature on the North American northeastern continental shelf. Elementa: Science of the Anthropocene 5:48, http://doi.org/10.1525/elementa.240.
Tulloch, V.J., É.E. Plagányi, C. Brown, A.J. Richardson, and R. Matear. 2019. Future recovery of baleen whales is imperiled by climate change. Global Change Biology 25:1,263–1,281, https://doi.org/10.1111/gcb.14573.
Vanderlaan, A.S.M., R.K. Smedbol, and C.T. Taggart. 2011. Fishing-gear threat to right whales (Eubalaena glacialis) in Canadian waters and the risk of lethal entanglement. Canadian Journal of Fisheries and Aquatic Sciences 68(12):2,174–2,193, https://doi.org/10.1139/f2011-124.
Zakardjian, B.A., J. Sheng, J.A. Runge, I. McLaren, S. Plourde, K.R. Thompson, and Y. Gratton. 2003. Effects of temperature and circulation on the population dynamics of Calanus finmarchicus in the Gulf of St. Lawrence and Scotian Shelf: Study with a coupled, three-dimensional hydrodynamic, stage-based life history model. Journal of Geophysical Research 180(C11), https://doi.org/10.1029/2002JC001410.